|
How to Request Testing of Anti-Tuberculosis Vaccine
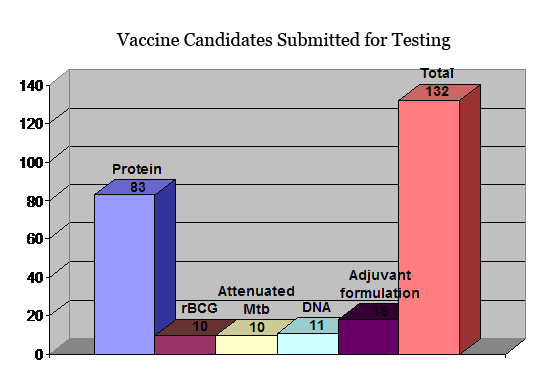
Investigators that wish to submit candidate anti-tuberculosis vaccines for testing in animal models of tuberculosis need to complete and submit a Vaccine Testing Application. An Expert Scientific Panel established by NIH will review all applications. Vaccine candidates selected for testing will first be evaluated in the murine model of tuberculosis. Vaccine candidates demonstrating protection in the murine model will be further tested for protective efficacy against tuberculosis in the guinea pig model. Investigators submitting requests for vaccine testing must provide written justification of why their candidate vaccine should be evaluated. Criteria used to select vaccines for testing include evidence of T or B cell response to the antigen, lack of toxicity, preliminary protection assays, the need for diversity in candidate vaccines, evidence of adjuvant activity, or evidence of attenuation for live candidate vaccines. It is important that forms are completed in full and detailed information regarding a vaccine's potential to protect against tuberculosis is provided. Lack of appropriate information may delay approval and testing. All information provided to the contract will be treated as confidential.
|


